
Advantages of H2
Hydrogen in the energy transition
Hydrogen in the energy transition
Recovering energy from previously stored renewable or low-carbon dihydrogen is done in two ways:
- either in the form of heat via its direct combustion with oxygen;
- or in the form of electricity via a fuel cell (PaC).
In both cases, the overall reaction only produces water and the energy produced can be used in various ways. Hydrogen is assigned three essential objectives for a successful energy transition.
Hydrogen to decarbonate transport
The challenges for mobility are considerable, because the hydrogen solution applied to clean mobility using direct combustion or the fuel cell, makes it possible to considerably reduce emissions.
Hydrogen in transport: a few figures
A diesel vehicle produces between 40 and 45 tonnes of CO2 over its lifetime, a hydrogen vehicle produced by reforming just over 35 tonnes, and a hydrogen vehicle produced by renewable electrolysis less than 15 tonnes.
Hydrogen-powered cars have at best a 74% lower carbon impact than traditional internal combustion vehicles
Energy Transition with SotayTank
Energy Transition with SotayTank
Over time, SotayTank has imagined evolving the fiberglass bottle, which is currently used for the resin in our water softeners, into a high-end “Type IV” composite product intended for other sectors. And in particular the energy storage market.
“It is a question of designing bottles in such a way as to be able to accommodate Hydrogen. Reservoirs to be integrated into the design of energy transport, following the principle of high pressure storage in gaseous form.
As the potential of hydrogen as a clean, renewable and scalable energy source becomes clear, opinions differ in the market as to how best to store the large volumes of H2 needed for various energy applications.
Fiberglass Cylinders, Carbon Fiber Hydrogen Tank
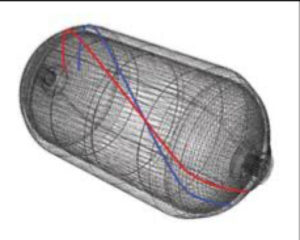
Storing renewable. energy in the form of hydrogen efficiently
For hydrogen to truly become the energy vector of tomorrow, it must be available at all times. In this context, developing effective storage and distribution methods is a crucial issue. Today, hydrogen storage remains the most satisfactory solution pending stable technical and economic specifications from manufacturers. There are three different storage strategies depending on the state of hydrogen (solid, liquid and gaseous). Its packaging in gaseous form nevertheless remains the most promising option. Storage in the form of compressed gas at very high pressures currently appears to be the solution offering the best compromise in terms of mass and volumetric density.
Progress regarding storage mode and pressures has been made by moving from 200 bars to 350 bars in the case of cylinders distributed in industry.
NPROXX’s Type IV 500 bar pressure vessel is now certified. It allows customers around the world to transport and store hydrogen in large quantities.
Now, developments are turning to tanks that can withstand pressures of 700 bar.

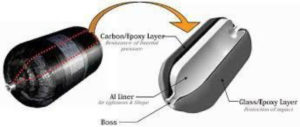
For easier and more efficient transport, hydrogen is stored in tanks or cylinders made of “type IV” composite materials (mainly carbon fibres) with a polymer liner (thermoplastic or thermosetting). In this case, the liner does not support the load, it only ensures the hydrogen seal. The special properties of the polymers make it possible to considerably extend the fatigue life of the tank and their low density makes it possible to reduce the total mass of the assembly. This combination makes it possible to reach high pressure levels comparable to those of type III tanks (700 bar). Type IV hydrogen storage is currently the most advanced technology.

Although the development of type IV tanks is currently the most satisfactory solution, taking into account all the technical and economic specifications formulated by manufacturers, an incorrectly sized tank can have very detrimental consequences in terms of safety, reliability, performance and cost. To improve the performance and durability of the tanks, progress and various tests are still necessary.
It is in this context that the development investment within the company SotayTank takes place. We have studied the storage of hydrogen in type IV tanks, made of carbon/epoxy composites for applications in the field of energy storage and transport. Our objective is to identify the specific damage mechanisms of the various tank constituents, to determine their durability under normal service conditions (excluding accidents/incidents) and to provide feedback on technologies, design, development and characterization tools. see qualification of these tanks.
To achieve these objectives, different steps are necessary:
- Characterize the mechanical and physical behavior of the materials used,
- Monitor the evolution of damage during quasi-static loading as well as durability in extreme temperatures (-40°C to 85°C),
- Perform multi-scale simulations to develop databases related to the physical properties of materials and their damage,
- Provide design offices with behavior laws and sizing criteria for the simulation of complete reservoirs.
This entire process is presented in this Investment Aid Project.
Our research and development activity is to work in particular on the mechanical strength of the materials making up these bottles over time. We must carry out accelerated fatigue tests using very high pressure filling and permeability cycles to ensure that they are perfectly sealed. All of this research will lay the scientific foundations for the behavior of materials and will help determine the criteria for the design of reservoirs.
Thanks to this research work, SotayTank becomes a decisive player in defining the safety standards that must be implemented to ensure maximum safety for the end user.
Another area of research for SotayTank is the development of bottle control technologies during their use. This step is also essential for user safety and consists of ensuring that there are no defects such as microcracks. For this, we must obtain from researchers, non-destructive testing methods such as acoustic emission to detect this type of anomaly.
Hydrogen is the lightest element, and arguably the most powerful of all. Given these two elements, supplying a significant amount of hydrogen to a fuel cell or a combustion engine is a major challenge. Hydrogen must therefore be stored at high density in order to contain enough fuel in a reasonable space to power a fuel cell in a mobile application such as a truck or a train. The two most accessible ways to achieve this are by storing H2 as a highly compressed gas or cryogenic liquid.
Both approaches have advantages and disadvantages. Due to its greatly reduced volume, liquid storage can work better when large quantities are transported. For example, cryogenics is often considered for transporting large volumes of hydrogen, as seen with the distribution of natural gas from the Middle East in giant tankers.
However, this option does not offer an adaptable solution to the modern diversity of hydrogen use methods. At SotayTank, we believe that the battle for daily hydrogen storage is already, and will continue to be, won by high pressure gas, stored in strong and lightweight carbon fiber Type IV pressure vessels. .
The hydrogen car
The hydrogen car
The hydrogen engine
The physico-chemical characteristics of hydrogen make it a good candidate for use as a fuel in a “gasoline” type spark ignition engine. The main advantage lies in the environmental balance: combined with oxygen, the combustion of hydrogen produces mainly water and heat and releases only nitrogen oxides (NOx). However, this solution requires specific adaptations to obtain very high efficiency and very low NOx emissions. In particular, it is necessary to exploit different properties of hydrogen such as its ability to burn quickly in a very lean mixture.
The use of hydrogen in an internal combustion engine can benefit from the latest advances in the heat engine and coupling with a hybrid powertrain. Thus, based on more robust and mature technologies than those currently used for fuel cells, it would be possible to achieve efficiency greater than 50%. This could be a transition solution to the fuel cell since it allows to start the validation of the entire hydrogen production and distribution chain using existing industrial production tools.
The fuel cell in electric cars
For the long term, car manufacturers are also interested in fuel cells (or Fuel Cell), as electricity generators for electric vehicles. This is in order to complete the solutions of battery-powered electric vehicles, which today suffer from the limitation in autonomy and the recharging time of these batteries. The hydrogen is then used to power a fuel cell — which produces electricity — to operate the electric motor that drives the vehicle. Hydrogen is one of the best energy carriers for fuel cells today in terms of energy performance and emissions. Their efficiency is generally greater than 50% over a wide operating range, which represents an interesting advantage compared to a current petrol engine.
Powered by a mixture of air and hydrogen, the cell converts the chemical energy of hydrogen into electrical energy following the reverse principle of electrolysis. By reacting hydrogen with oxygen from the air on the electrodes (thin membranes covered with a catalyst, platinum), fuel cells make it possible to produce electricity with no emissions other than carbon. water vapour. The principle dates back to 1839! It has long been used to generate electricity on board rockets.
From hydrogen to electricity
Hydrogen for the storage of electricity and its injection into the networks
The storage of energy in the form of hydrogen makes it possible to overcome the intermittency of renewable energies (wind and solar) by optimizing the electricity production capacity
As part of the development of a renewable electricity mix, electrolysis makes it possible, when the network has a surplus (i.e. when the production of electricity is greater than its consumption), to store hydrogen on a short or long time depending on the needs.
In the case of a deficit network on the contrary, the available hydrogen can be reused in a fuel cell to produce electricity
Hydrogen can also be injected directly into gas networks
- on the one hand, to supply carbon-free energy to the industrial units concerned;
- on the other hand to contribute to the decarbonization of the industrial processes concerned by replacing the fossil fuels currently used: this is the case, for example, of the manufacture of steel which results from the reduction of iron ore
This reduction carried out today via coal could be done tomorrow by using decarbonated hydrogen.
Frequently Asked
Questions about HYDROGEN
-
Show answer to the question Green, grey, blue and yellow hydrogen: what are we talking about?
Green, grey, blue and yellow hydrogen: what are we talking about?
- Green hydrogen is made by electrolysis of water using electricity from renewable energy only;
- Gray hydrogen is manufactured by thermochemical processes using fossil sources as raw materials (coal or natural gas);
- Blue hydrogen is manufactured in the same way as gray hydrogen, except that the CO2 emitted during manufacture will be captured for reuse or storage;
- Yellow hydrogen, more specific to France, is produced by electrolysis like green hydrogen, but the electricity comes mainly from nuclear energy.
Ademe recently suggested changing the terminology. Hydrogen which was previously called “green” is now called “renewable”, “grey” hydrogen becomes “fossil”, and finally, “blue” and “yellow” hydrogens are grouped together under the name “low -carbon”.
-
Show answer to the question How is hydrogen stored?
How is hydrogen stored?
Dihydrogen has a very high mass energy density (1 kg of hydrogen contains as much energy as about 3 kg of petroleum) but a very low volume density. It must be transformed in order to be able to store it in a usable volume.
- By compressing it to 700 bar: 7 liters of hydrogen can thus contain as much energy as 1 liter of gasoline;
- By liquefying it to compress it further at a temperature of -253°C: 4 liters of liquid hydrogen are then equivalent to 1 liter of gasoline.
Densifying hydrogen makes it possible to operate at lower pressures but requires more energy, which makes it more expensive.
There are many storage methods (batteries, massive storage in saline cavities) depending on the intended use.
-
Show answer to the question How is hydrogen used?
How is hydrogen used?
Currently, hydrogen has two main uses: on the one hand, it serves as a feedstock for the production of ammonia (fertilizer) and methanol; on the other hand, it is used as a reagent in the processes of refining crude oils into petroleum products, fuels and biofuels.
The uses that can be made of it are nevertheless numerous, and hydrogen is promising for decarbonizing a certain number of sectors and supporting the energy transition.
-
Show answer to the question What future for hydrogen?
What future for hydrogen?
The deployment of decarbonated hydrogen is to be considered by the end of the decade, its full development being rather for the following one. It requires lifting a number of locks.
Lower costs
Green hydrogen is very expensive and can only be deployed if costs are reduced across the entire value chain, starting with the cost of producing renewable electricity (solar, wind) but also that of electrolysers or fuel cellsHow much does decarbonated hydrogen cost?
How much does decarbonated hydrogen cost?
Producing hydrogen from electrolysis today costs 2 to 3 times more than steam reforming and 2 times more expensive than reforming with CO2 capture. This route is currently reserved for specific uses such as electronics, which require a high level of purity.
The complexity of the value chain and the various transformations also involve yield cascades, sources of energy losses, which have the effect of increasing production costs.
At the same time, a relatively high CO2 price would reduce the cost gap with natural gas reforming. However, the increase in carbon taxation must be gradual and accompanied by public policies to support the poorest populations. -
Show answer to the question Where is the hydrogen found?
Where is the hydrogen found?
The main resources to produce dihydrogen H2 (which is called hydrogen by abuse of language) are water and hydrocarbons (coal, oil or gas).
- Indeed, each molecule of water is the result of the combination between an oxygen atom and two hydrogen atoms, according to the formula H2O.
- Hydrocarbons come from the combination of carbon and hydrogen atoms. This is for example the case of methane, the main constituent of natural gas whose formula is CH4, one of the simplest combinations for hydrocarbons
Hydrogen also exists naturally. The first natural sources of hydrogen were discovered at the bottom of the sea in the 1970s and more recently on land. But there is a long way to go before considering a profitable operation. Knowledge of the origin of the formation of this hydrogen and research into cost-effective production techniques still need to progress.
Once manufactured, the hydrogen must be stored and then transported to its place of distribution and use.
Interested in HYDROGEN ?
Let’s talk.




